Johnson & Johnson’s Covid-19 vaccine gets official recommendation from CDC
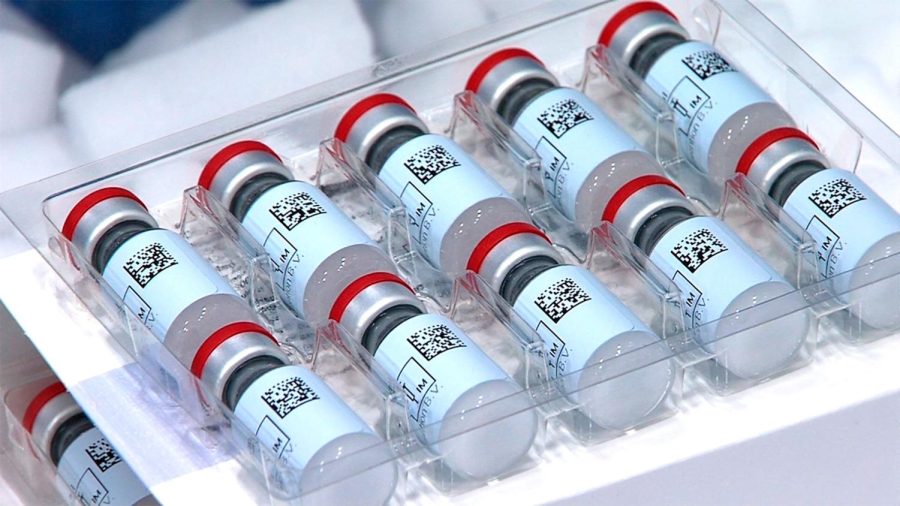
This Dec. 2, 2020 photo provided by Johnson & Johnson shows vials of the COVID-19 vaccine in the United States. The nation is poised to get a third vaccine against COVID-19, but health officials are concerned that at first glance the Johnson & Johnson shot may not be seen as equal to other options from Pfizer and Moderna. (Johnson & Johnson via AP)
March 1, 2021
(CNN) — Centers for Disease Control and Prevention vaccine advisers voted Sunday to recommend the Johnson & Johnson Covid-19 vaccine for the US, and CDC Director Dr. Rochelle Walensky almost immediately signed off on the recommendation.
It is the first of the three authorized Covid-19 vaccines that comes in a single dose.
“The Janssen vaccine has been shown to be safe and effective in preventing severe COVID-19 illness, hospitalization, and death,” Walensky said in a statement.
“This vaccine is also another important tool in our toolbox to equitably vaccinate as many people as possible, as quickly as possible,” she added.
“As a one-dose vaccine, people do not have to return for a second dose to be protected. In addition, this vaccine does not need to be kept in a freezer and can be stored at refrigerated temperatures — so it is easy to transport and store and allows for expanded availability in most community settings and mobile sites, as supply scales up.”
The US Food and Drug Administration authorized the J&J vaccine on Saturday.
The CDC’s Advisory Committee on Immunization Practices is a group of vaccine and public health experts that helps set guidelines for the CDC concerning the best practices with vaccinations. Members voted unanimously, with one recusal for a potential conflict of interest, to recommend the vaccine. They did not make any recommendations about specific groups who should receive the vaccine.
Now the federal government may then begin distributing the 3.9 million available doses of the vaccine, perhaps as soon as Monday.
“I just want to state explicitly how very grateful I am that we now have three highly effective vaccines,” said ACIP member Dr. Matthew Daley of the Institute for Health Research with Kaiser Permanente Colorado.
The company has pledged to have 20 million doses available by the end of March and 100 million doses by summer.
The vaccine, made by Johnson & Johnson’s Janssen vaccine arm, can be kept at regular refrigerator temperatures, which experts said would make it much easier to distribute than vaccines made by Moderna and Pfizer/BioNTech.
“During a pandemic, the data show that the best utilization of resources is to employ all available vaccines with acceptable vaccine efficacy. This will save cost and lives,” the CDC’s Dr. Sara Oliver told the ACIP meeting. A single-dose vaccine has an advantage, particularly in settings where a second dose “would be challenging.” For example, it could be used to help protect the homeless, people in the justice system, and those with limited access to health care like people who are homebound or live in rural areas, Oliver said.
The vaccine was tested in more than 44,000 people in the US, South Africa and Latin America. Globally, it was 66.1% effective against moderate to severe/critical Covid-19 at least four weeks after vaccination, according to an FDA analysis. In the US, it is considered 72% effective, and offered 86% protection against severe forms of the disease.
Overall, non-fatal serious adverse events were infrequent, according to the FDA’s analysis, and there were no reported cases of anaphylaxis following vaccination in the trial. There have been a small number of severe allergic reactions with the Moderna and Pfizer/BioNTech vaccines. For example, in the first week of the Pfizer vaccine rollout, there were only 29 cases out of 1.9 million doses administered, according to the CDC.
More research is needed to know for sure, but the FDA analysis also hinted that the J&J vaccine may help prevent asymptomatic infections.
A January study from the CDC showed that most coronavirus cases are spread by people without symptoms. If a vaccine prevented asymptomatic infection, it might help reduce opportunities to transmit the disease — not just keep the vaccinated from getting sick.
There is some concern among public health leaders that some people will think the J&J vaccine is “second class,” because its efficacy is lower than the Moderna and Pfizer vaccines, but the experts say it is important that people remember these are totally different vaccines.
The J&J vaccine was tested at a time when there were more variants in circulation. Tested in South Africa and in Brazil, an FDA analysis found that the majority of the Covid-19 cases in the J&J trials were due to variants that are thought to be more contagious.
“The Johnson & Johnson vaccine was tested against the South Africa variant in South Africa, tested against the variant in Brazil. The Moderna and Pfizer vaccines weren’t, we are not comparing the same thing,” said Dr. Ashish Jha, the dean of Brown University School of Public Health. “When you look at what we really compare about which is preventing hospitalizations and deaths, the Johnson & Johnson vaccine comes in at 100% once it’s had a chance to work.”
“This is a terrific vaccine and I would certainly, I would take it,” Jha said Sunday on CNN’s Inside Politics. “I would recommend it to my family and I think people who are worried about that headline number, I say ignore it because that’s not really what you care about.”
The three Covid-19 vaccines have not been compared head to head, so it would be impossible to know if one is better than the other.
“These are three highly efficacious vaccines,” said Dr. Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases. “I can tell you I have been fully vaccinated with one that was available. It was the Moderna. If I were not vaccinated now and I had a choice of getting a J&J vaccine now or waiting for another vaccine, I would take whatever vaccine would be available to me as quickly as possible.”
“We want to get as many people vaccinated as quickly and expeditiously as possible,” Fauci said Sunday on CNN’s State of the Union. “So this is good news because we have another very good vaccine in the mix.”
The-CNN-Wire
™ & © 2021 Cable News Network, Inc., a WarnerMedia Company. All rights reserved.